Which of the Following Best Describes a Single-replacement Reaction
HINT you will need to perform two calculations and then add up the results. Helpful 0 Not Helpful 1 Add a Comment.
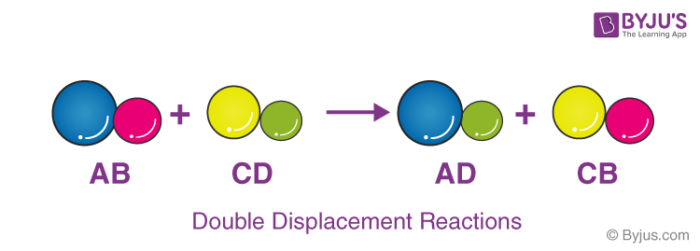
What Is Double Displacement Reaction Chemistry Question
Definition of single replacement or single displacement reactions.

. Which of the following best represents the reaction between sulfuric acid and calcium hydroxide. A single replacement reaction occur when an element in a substance is replaced by other element. Which of the following best describes a single replacement reaction.
Propane Is a hydrocarbon a compound composed only of carbon and hydrogen. Na s H 2 O l. The the following reaction is.
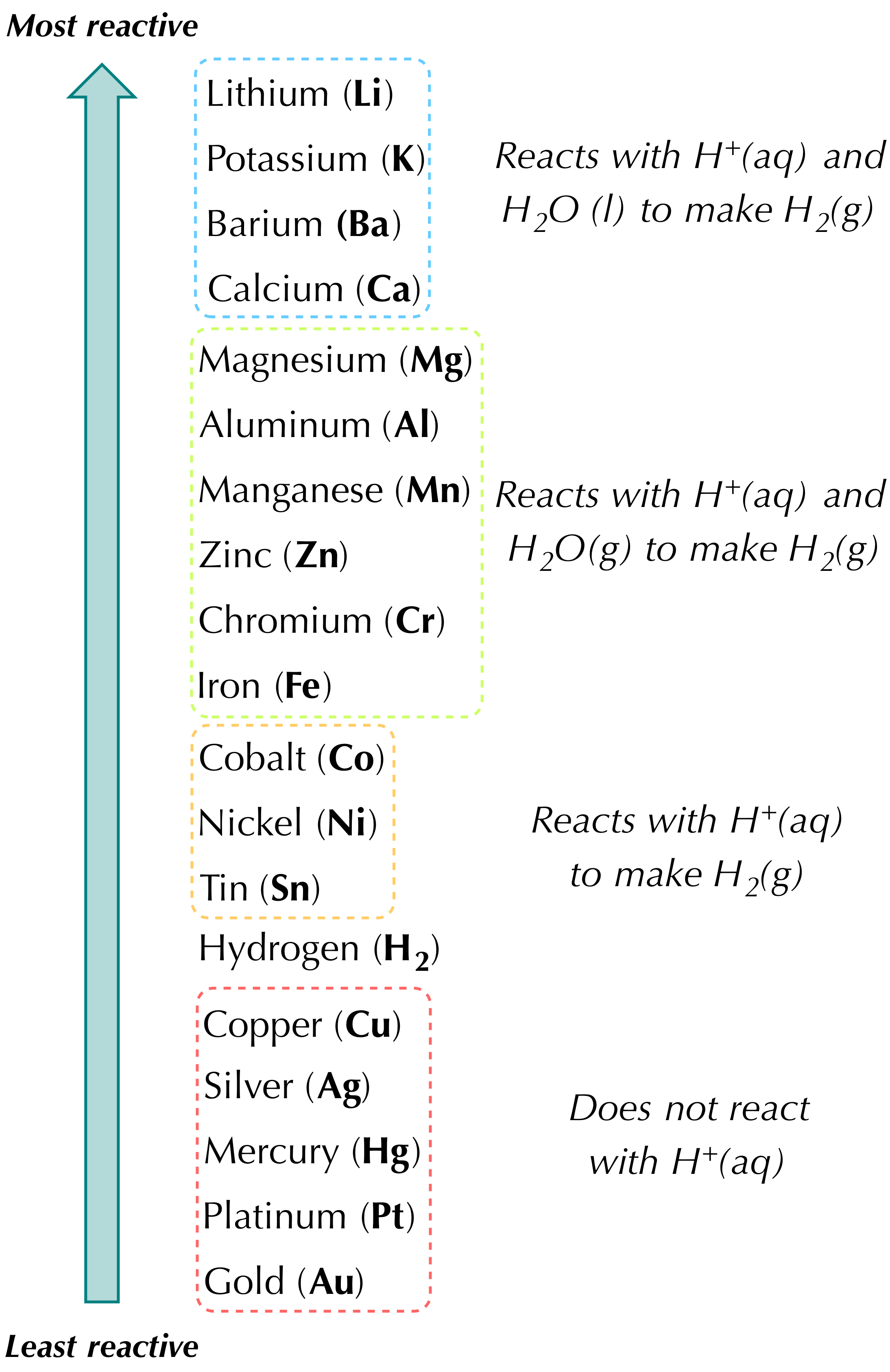
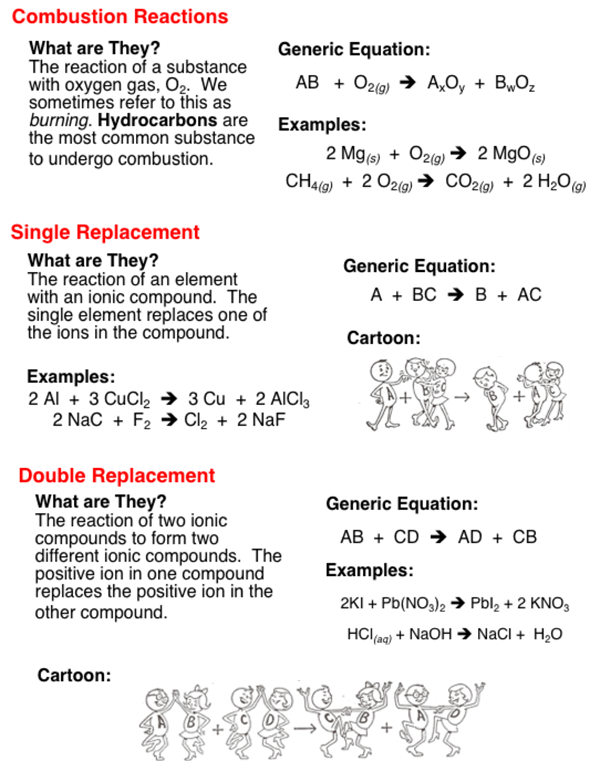
A single-replacement reaction also called a single-displacement reaction is one in which a pure element and a compound react chemically so that the products include another pure element and a different compound via exchanging of two species. Predicting and determining the products using the reactivity series. F H2 I2 2HI G 2NaCl 2Na Cl2 H NaF HCl HF NaCl.
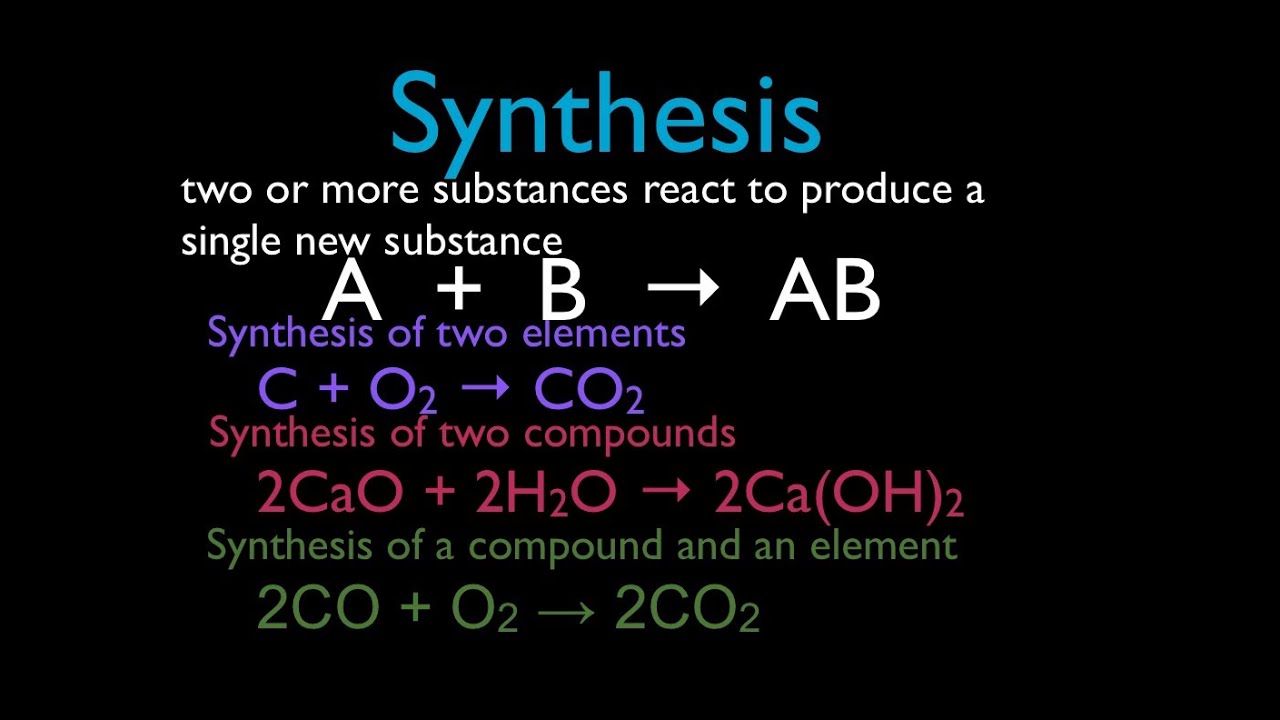
An essay that includes a synthesis of two sources as well as the authors opinion is a synthesis and response essay. Two elements combine to form a compound one element takes the place. 2 question The reaction 2Mgs O2g -- 2MgOs is a Group of answer choices double-replacement reaction.
Which of these reactions shows simple chemical decomposition. How much heat is needed for this total process. A single substance breaks down into more than one substance.
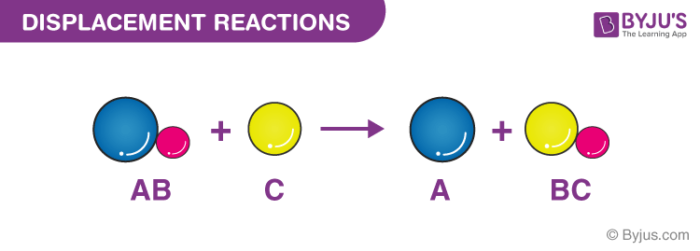
The positive hydrogen ion on the Chlorine has been replaced by a positive sodium ion on the Chlorine. Which reaction type best describes the. This is a double replacement reaction that is also a neutralization It is a double replacement because the reaction starts with two compounds and ends with two compounds where the positive and negative ions have changed places.
F H2SO4 CaOH2 CaSO4 H2O. What best describes a synthesis and response essay. This is the best answer based on feedback and ratings.
Sodium metal reacts with water in the following single replacement reaction. Consider the following equation. Two elements combine to form a compound one element takes the place of another in a compound two elements switch places in a compound a compound breaks into separate elements.
In other words it is where an element reacts with a compound and replaces one component of the compound. Ice with a mass of 15 grams is melted AND then the temperature of the now liquid water is raised from 0 degrees Celsius up to 95 degree Celsius. The best predictor of major expenditures that do not have status or symbolic.
Which of the following best describes this type of chemical reactiona. While the Sodium ion on. NaOH aq H 2 g Determine the limiting reactant and mass of hydrogen gas produced when 20g of sodium is added to 100g of water.
2Na s 2H2O l 2NaOH aq H2 g limi. Balance polyatomic ions as a single unit check each reactant and product to verify the coefficients. Zns CuSO4aq - Cu and ZnSO4.
Describe the characteristic electron-domain geometry of each of the following numbers of electron domains about a central atom. Which of the following best describes a single replacement reaction. Combination reaction C single-replacement reaction D double-replacement reaction E neutralization reaction.
Which of the following best describes a single replacement reaction. BaCO3 -- BaO CO2. 2 When magnesium is burned in the presence of oxygen it produces magnesium oxide according to the following chemical equation.
A substance combines with oxygen and releases energy in tge form of heat and light. Which of the statements below best describes the following reaction. Which of the following best describes a decomposition reaction.
What are the products from the following single-replacement reaction. What type of chemical reaction is illustrated in the following example. See answer 1 Best Answer.
HClaq KOHaq KClaq H2Ol. Mathrm Zn s2 mathrm HCl a q rightarrow mathrm ZnCl_ 2 a qmathrm H_ 2 g The reaction can be made to occur more slowly by 1 raising the temperature and using a single piece of zinc rather than powdered zinc of the same mass 2 lowering the. It Is 8182 carbon and 1818 hydrogen.
Two elements combine to form a compound. Which of the statements below best describes the following reaction. C Single replacement D Combustion.

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube
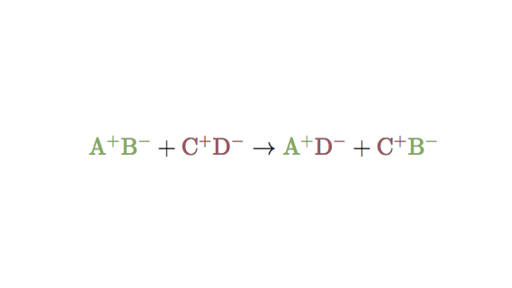
Double Replacement Reactions Double Displacement Article Khan Academy
Ch104 Chapter 5 Chemical Reactions Chemistry

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube

What Is The Difference Between Single Displacement Reaction Double Displacement Reaction Chemistry Question
Single Replacement Reactions Article Khan Academy

Paige Wolf 10 3typesofequations Pdf Types Of Reactions Name Paige Wolf Chem Worksheet 10 3 Balance The Following Reactions Classify The Reactions Course Hero
Single Replacement Reactions Article Khan Academy

Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube

Single Replacement Reaction Definition And Examples

Replacement Reaction Ck 12 Foundation
What Is The Chemical Reaction Of Zinc And Copper Ii Sulfate Quora
Fe2o3 2al Al2o3 2fe The Reaction Is An Example Of A A Combination Reaction B Double Displacement Reaction C Decomposition Reaction D Displacement Reaction Chemistry Q A

Double Replacement Reaction Is A Type Of Chemical Reaction Where Two Compounds React And The Positive Negative I Positive And Negative Positivity Negative Ions

Chemical Reaction Ck 12 Foundation
Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube

Comments
Post a Comment